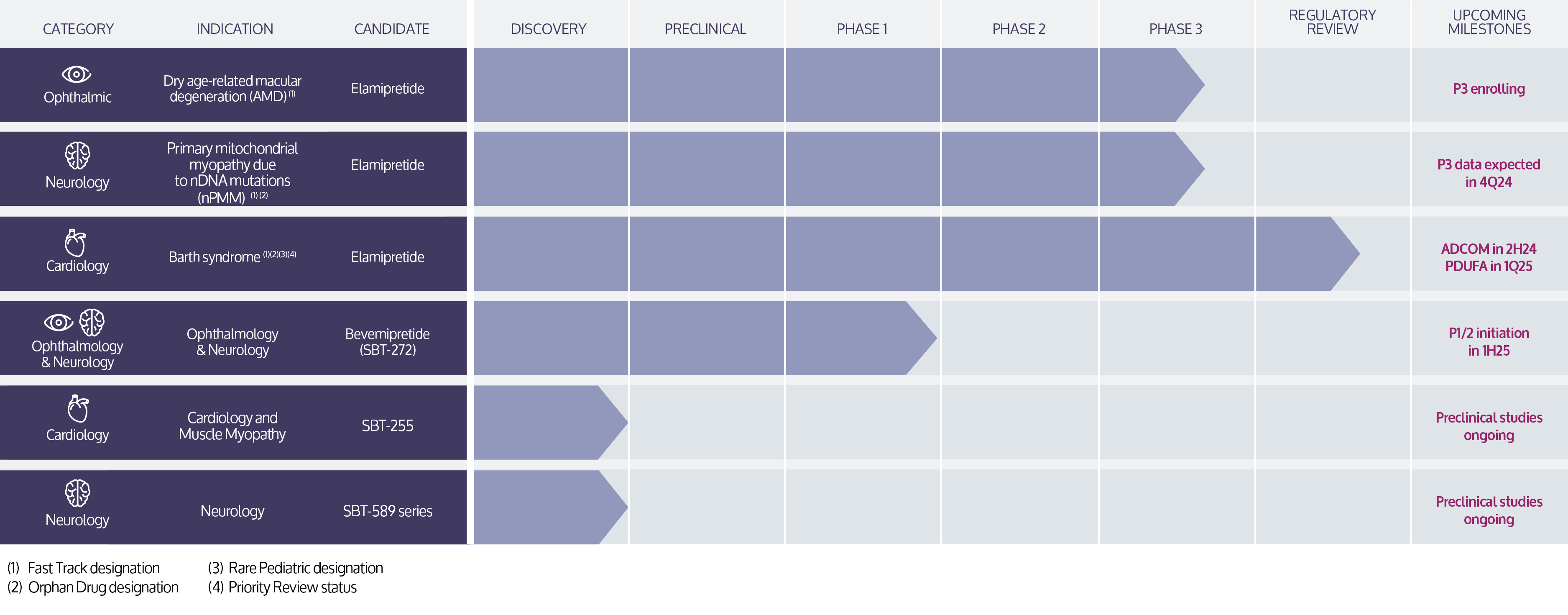
Stealth’s lead investigational product candidate, elamipretide, is a peptide compound that readily penetrates cell membranes, and targets the inner mitochondrial membrane where it binds reversibly to cardiolipin.13 In preclinical or clinical studies, we have observed that elamipretide increases mitochondrial respiration, improves electron transport chain function and ATP production and reduces formation of pathogenic ROS levels.13-19 This elamipretide-cardiolipin association has been shown to normalize the structure of the inner mitochondrial membrane, thereby improving mitochondrial function.13 Functional benefit is achieved through improvement of ATP production and interruption and potential reversal of damaging oxidative stress.13 We are investigating elamipretide in late stage clinical studies in ophthalmic diseases entailing mitochondrial dysfunction, such as dry AMD, rare neuromuscular disorders, such as primary mitochondrial myopathy, and rare cardiomyopathies, such as Barth syndrome. We are evaluating our second-generation clinical-stage candidate, Bevemipretide (SBT-272), for rare neurological disease indications, such as amyotrophic lateral sclerosis, and have a deep pipeline of novel compounds under evaluation for rare neurological and cardiac disease indications.
Healthy & Unhealthy