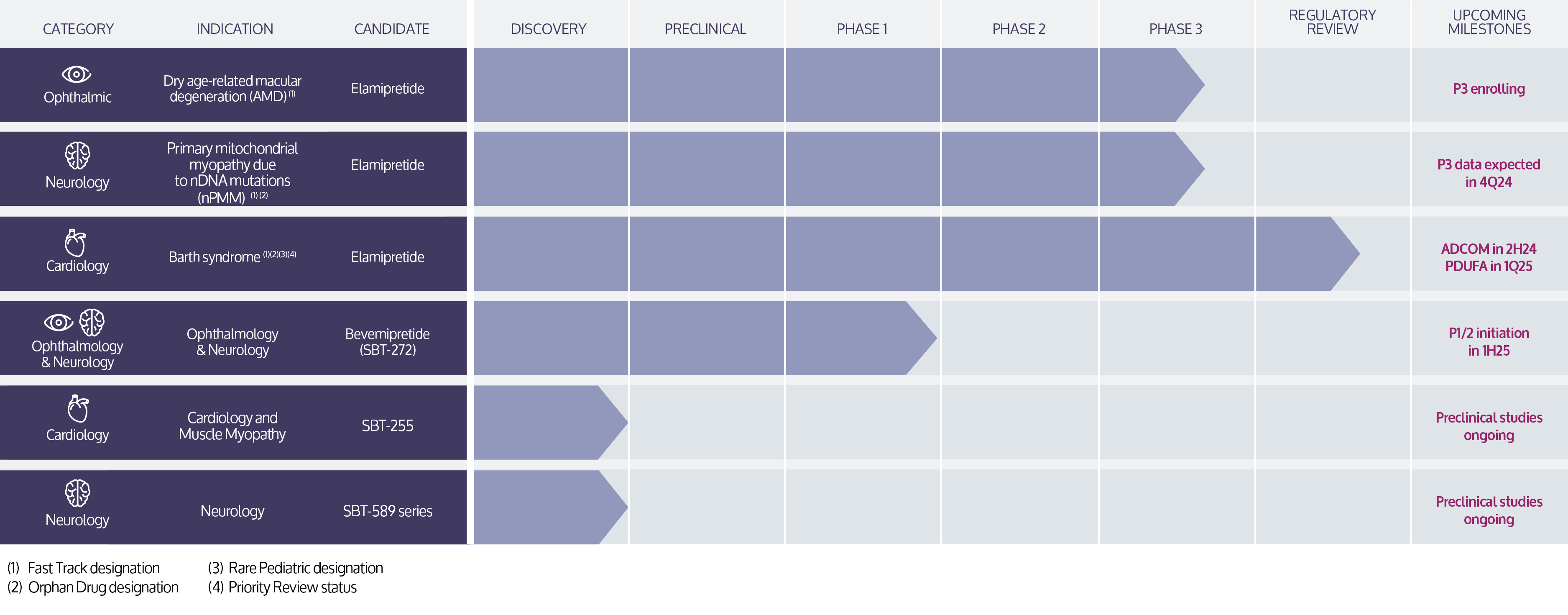
Stealth’s lead investigational product candidate, elamipretide, is a peptide compound that readily penetrates cell membranes, and targets the inner mitochondrial membrane where it binds reversibly to cardiolipin.13 In preclinical or clinical studies, we have observed that elamipretide increases mitochondrial respiration, improves electron transport chain function and ATP production and reduces formation of pathogenic ROS levels.13-19 This elamipretide-cardiolipin association has been shown to normalize the structure of the inner mitochondrial membrane, thereby improving mitochondrial function.13 Functional benefit is achieved through improvement of ATP production and interruption and potential reversal of damaging oxidative stress.13 We are investigating elamipretide in late stage clinical studies in ophthalmic diseases entailing mitochondrial dysfunction, such as dry AMD, rare neuromuscular disorders, such as primary mitochondrial myopathy and Duchenne muscular dystrophy, and rare cardiomyopathies, such as Barth syndrome. We are evaluating our second-generation clinical-stage candidate, SBT-272, for rare neurological disease indications, such as amyotrophic lateral sclerosis.
Healthy & Unhealthy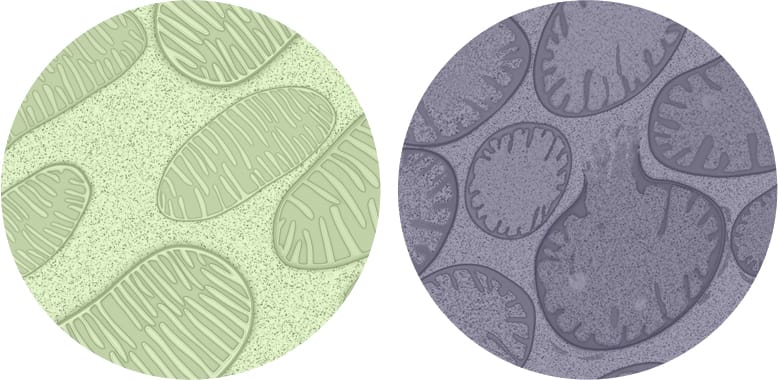

.graph-mobile { display: none; } @media (max-width: 767px) { .graph-desktop { display:none !important; } .graph-mobile { display:block !important; } }
{
“@context”: “https://schema.org/”,
“@type”: “Drug”,
“activeIngredient”: “Elamipretide”,
“administrationRoute”: “Subcutaneous”,
“breastFeedingWarning”: “Elamipretide is an investigational drug pending FDA approval. There is no information available on the safety and efficacy of elamipretide use in pregnant or breastfeeding women.”,
“clinicalPharmacology”: “Elamipretide is a novel tetrapeptide drug that repairs defects in mitochondrial function by binding to cardiolipin, resulting in the restoration of mitochondrial membrane potential, improved ATP production, normal calcium flux, and reduced superoxide generation.”,
“dosageForm”: “Injection”,
“drugClass”: “Neurologics, Other”,
“isAvailableGenerically”: “true”,
“isProprietary”: “true”,
“legalStatus”: “Elamipretide is an investigational drug being developed to treat mitochondrial diseases, yet to be approved by the FDA, but has received orphan drug status.”,
“prescriptionStatus”: “Elamipretide is an investigational drug being developed to treat mitochondrial diseases, yet to be approved by the FDA, but has received orphan drug status.”,
“nonProprietaryName”: [
“Elamipretide”,
“H-D-Arg-Tyr(2,6-diMe)-Lys-Phe-NH2”,
“D-Arginyl-2,6-dimethyl-L-tyrosyl-L-lysyl-L-phenylalaninamide”
],
“proprietaryName”: [
“Bendavia”,
“ELA 4”,
“Elamipretide-hydrochloride”,
“Elamipretide-hydrochloride – Stealth Biotherapeutics”,
“MTP 131”,
“MTP-131”,
“MPT131”,
“Ocuvia”,
“SBT-31”,
“SPI-31”,
“SS-31”,
“RX-31”,
“RX31”,
“SS31”,
“SZETO-SCHILLER PEPTIDE”,
“SBT-272”
],
“pregnancyWarning”: “Elamipretide is an investigational drug pending FDA approval. There is no information available on the safety and efficacy of elamipretide use in pregnant or breastfeeding women.”,
“warning”: “Elamipretide is an investigational drug pending FDA approval. There is no information available on contraindications, warnings and precautions for the use of elamipretide. Common side effects of elamipretide include: Headaches, dizziness, abdominal pain, flatulence, and mild redness or itching at the injection site. Call your doctor immediately if you experience any of the following symptoms or serious side effects while using this drug: Serious heart symptoms, Including fast or pounding heartbeats, fluttering in your chest, shortness of breath, and/or sudden dizziness, severe headache, confusion, slurred speech, severe weakness, vomiting, loss of coordination, feeling unsteady, severe nervous system reactions with very stiff muscles, high fever, sweating, tremors, feeling like you might pass out, serious eye symptoms, including blurred vision, tunnel vision, eye pain or swelling, or seeing halos around lights. This is not a complete list of all side effects or adverse reactions that may occur from the use of this drug. Call your doctor for medical advice about serious side effects or adverse reactions. You may also report side effects or health problems to the FDA at 1-800-FDA-1088.”,
“brand”: “Stealth BioTherapeutics Inc.”,
“category”: “Investigational Therapy”,
“medicineSystem”: “WesternConventional”,
“relevantSpecialty”: [
“Neurology”,
“Cardiology”,
“Endrocrinology”,
“Gastroenterology”,
“Ophthalmology”,
“Neuro-ophthalmology”,
“Mitochondrial medicine”
],
“study”: [
“https://clinicaltrials.gov/study/NCT05162768”,
“https://stealthbt.com/wp-content/uploads/TAZPOWER-Infographic_Updated-4.6.18.pdf”,
“https://stealthbt.com/wp-content/uploads/1907_StealthBio_Phase2_Infographic_FINAL-converted-1.pdf”,
“NuPOWER (nuclear DNA mutations nPMD)”,
“TAZPOWER (Barth Syndrome)”,
“ReCLAIM-2 (Age-Related Macular Degeneration)”,
“SBT-272 Phase 1”
],
“hasAdultConsideration”: “HealthcareConsideration”,
“description”: “Stealth’s lead investigational product candidate, elamipretide, is a peptide compound that readily penetrates cell membranes, and targets the inner mitochondrial membrane where it binds reversibly to cardiolipin. In preclinical or clinical studies, we have observed that elamipretide increases mitochondrial respiration, improves electron transport chain function and ATP production and reduces formation of pathogenic ROS levels. This elamipretide-cardiolipin association has been shown to normalize the structure of the inner mitochondrial membrane, thereby improving mitochondrial function. Functional benefit is achieved through improvement of ATP production and interruption and potential reversal of damaging oxidative stress. We are investigating elamipretide in late stage clinical studies in ophthalmic diseases entailing mitochondrial dysfunction, such as dry AMD, rare neuromuscular disorders, such as primary mitochondrial myopathy and Duchenne muscular dystrophy, and rare cardiomyopathies, such as Barth syndrome. We are evaluating our second-generation clinical-stage candidate, SBT-272, for rare neurological disease indications, such as amyotrophic lateral sclerosis.”,
“identifier”: [
“(2S)-6-Amino-2-[[(2S)-2-[[(2R)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-(4-hydroxy-2,6-dimethylphenyl)propanoyl]amino]-N-[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]hexanamide”,
“736992-21-5”,
“11764719”,
“87GWG91S09”,
“D10925”,
“DTXSID50471988”
],
“mainEntityOfPage”: “https://stealthbt.com/programs-pipeline/”,
“sameAs”: [
“https://en.wikipedia.org/wiki/Elamipretide”
],
“subjectOf”: [
“https://stealthbt.com/wp-content/uploads/In-vivo-mitochondrial-ATP-production-is-improved-in-older-adult-skeletal-muscle-after-a-single-dose-of-elamipretide-in-a-randomi.pdf”,
“https://stealthbt.com/wp-content/uploads/Phase-1-Clinical-Trial-of-Elamipretide-in-Intermediate-Age-Related-Macular.pdf”,
“https://stealthbt.com/wp-content/uploads/Phase-1-Clinical-Trial-of-Elamipretide-in-Dry-Age-Related-Macular-Degeneration-and-Noncentral-Geographic-Atrophy-ReCLAIM-NCGA-Study.pdf”,
“https://stealthbt.com/wp-content/uploads/Elamipretide-Poster-ARVO-Final_29Apr-002.pdf”,
“https://stealthbt.com/wp-content/uploads/Priyatham-Mettu-ARVO-2019-poster.pdf”,
“https://stealthbt.com/wp-content/uploads/Mechanisms-of-mitochondrial-dysfunction-and-their-impact-on-age-related-macular-degeneration.pdf”,
“https://stealthbt.com/wp-content/uploads/Association-of-Ellipsoid-Zone-Integrity-and-Treatment-Response-in-Non-Neovascular-AMD-Treated-With-Subcutaneous-Elamipretide.pdf”,
“https://stealthbt.com/wp-content/uploads/Barth-Syndrome-Elamipretide-2020.pdf”,
“https://stealthbt.com/wp-content/uploads/Thompson-and-Vernon_Genetic-in-Medicine_2020_TAZPOWER-online.pdf”,
“https://stealthbt.com/wp-content/uploads/SCWD-2020_Metabolomics-poster_v3_11.24.2020.pdf”,
“https://stealthbt.com/wp-content/uploads/UMDF_2019_TAZPOWER_Cardiolipin_Ratio_Results.pdf”,
“https://stealthbt.com/wp-content/uploads/UMDF_2019_TAZPOWER_Study_Design.pdf”,
“https://stealthbt.com/wp-content/uploads/Barth-Vernon.pdf”,
“https://stealthbt.com/wp-content/uploads/Vaz-article-re-MLCL.pdf”,
“https://stealthbt.com/wp-content/uploads/cardiolipin-Barth.pdf”,
“https://stealthbt.com/wp-content/uploads/cardiolipin-barth-2013.pdf”,
“https://stealthbt.com/wp-content/uploads/Elamipretide-Improves-Mitochondrial-Function-in-the-Failing-Human-Heart.pdf”,
“https://stealthbt.com/wp-content/uploads/Senger-Syndrome-AEPCC-poster-1.pdf”,
“https://stealthbt.com/wp-content/uploads/ACC_2020_TAZPOWER-Poster_v15.pdf”,
“https://stealthbt.com/wp-content/uploads/MDA-2022-Dystrophin-poster-PRINT-100_3X4-FINAL.pdf”,
“https://stealthbt.com/wp-content/uploads/NEALS2020_66_Poster-TDP43-SBT272_PHO_UploadID-125649-v2-2.pdf”,
“https://stealthbt.com/wp-content/uploads/NEALS-2019_Keefe_Poster_9.27.2019_FINAL.pdf”,
“https://stealthbt.com/wp-content/uploads/The-mitochondrial-targeted-drug-SBT-272-attenuates-dopaminergic-neuron-loss-alpha-synuclein-burden.pdf”,
“https://stealthbt.com/wp-content/uploads/Siegel_et_al-2013-Aging_Cell.pdf”,
“https://stealthbt.com/wp-content/uploads/Elamipretide-in-RAS-Circ-CV-Interventios-2017_Textor.pdf”
]
}